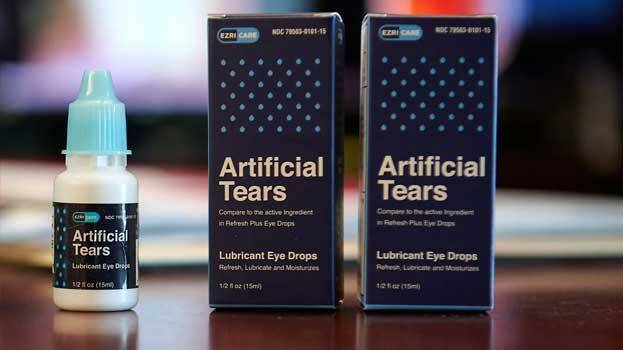

NEW DELHI: US drug regulatory authority issued a warning after it found high levels of drug-resistant bacteria in India-made eye drops. ‘Artificial tears’, made by Tamil Nadu-based Global Pharma has come under scanner after the presence of Pseudomonas aeruginosa bacteria. US Health authorities have raised concern over the issue.
The Pharma company had withdrawn the eye drops from the market in February after Centre for Disease Control and Prevention (CDC) report mentioned the death of 3 people and 8 others losing vision due to the usage of the medicine. The bacterial strain has been found after the report was published.
According to US drug regulatory authority, the usage of contaminated eye drops will lead to permanent vision loss and severe infections leading to death. It also states that antibiotics are not effective against this strain of bacteria.
Recently, India-made cough syrups hit the headlines after the death of 18 children in Uzbekistan and 66 children in Gambia. The incident also raised questions on the efficacy of India-made medicines.
Meanwhile, PV Vijayalakshmi, Director of Drugs Control in Tamil Nadu said that no drug-resistant bacteria has been found in the eye drops despite examining different samples from various batches.