
NEW DELHI: The Indian Medical Association (IMA) has criticized the arrest of Dr. Praveen Soni in the case of the death of 14 children after consuming cough medicine. The IMA responded by asking what the doctor did wrong by prescribing the medicine approved by the government. The IMA alleged that this is a perfect example of the legal ignorance of the officials and that it is a hasty action to hide the shortcomings of the government and the drug manufacturers.
"Why is the doctor being held responsible. The doctor prescribed a cough syrup supplied by a government hospital. It is not the doctor’s duty to test the purity of a government-supplied medicine. That responsibility lies with the drug controller and the government,” said Dr. Dilip Bhanushali, President of the Indian Medical Association (IMA). The IMA also clarified that it will counter the threat against health workers.
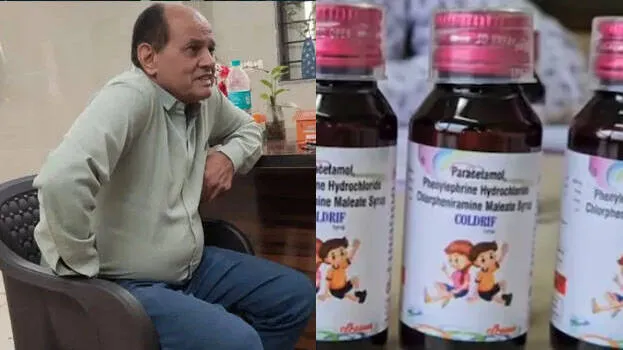
The doctor who prescribed the cough syrup that caused the death of the children has been arrested. Praveen Soni is the doctor at the clinic who examined most of the children who died. Police say the doctor prescribed Coldrif syrup to the children. Coldrif syrup is manufactured by Srisan Pharmaceuticals, based in Kancheepuram, Tamil Nadu. The police have registered a case against them.
The government had earlier banned the sale of Coldrif. Sample testing revealed that the medicine contained 48.6 percent diethylene glycol, a highly toxic substance. The Tamil Nadu Drug Control Directorate has declared the syrup, which was tested at a drug testing laboratory in Chennai, to be of substandard quality. As a precautionary measure, the authorities have banned the sale of Coldrif and another cough syrup, 'Nextro-DS'.
So far, 14 children have died after consuming Coldrif syrup across the country. The syrup has also been banned in Telangana. 'Coldrif' has been banned in Kerala as well. Veena George informed that the action was taken following reports from outside Kerala that a problem was found in the SR13 batch of Coldrif syrup.